
Chemistry, 18.06.2021 20:40 KylaChanel4756
A solution of permanganate is standardized by titration with oxalic acid, . To react completely with mol of oxalic acid required 28.18 mL of permanganate solution. The unbalanced chemical equation for the reaction in acidic solution is Determine the concentration of the permanganate solution in molarity. g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2


Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
A solution of permanganate is standardized by titration with oxalic acid, . To react completely with...
Questions



Physics, 09.12.2019 12:31


Mathematics, 09.12.2019 12:31

History, 09.12.2019 12:31

Mathematics, 09.12.2019 12:31






Mathematics, 09.12.2019 12:31



History, 09.12.2019 12:31

Mathematics, 09.12.2019 12:31



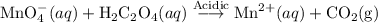
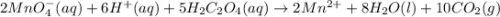
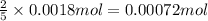 of permanganate solution
of permanganate solution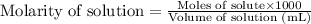 .....(1)
.....(1)


