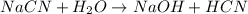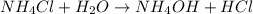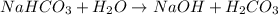Chemistry, 18.06.2021 02:00 alananicoleee
State whether 0.1 M solutions of each of the following salts are acidic, basic, or neutral. Explain your reasoning for each by writing a balanced net ionic equation to describe the behavior of each nonneutral salt in water: NaCN, KNO3, NH4Cl, NaHCO3, and Na3PO4.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
State whether 0.1 M solutions of each of the following salts are acidic, basic, or neutral. Explain...
Questions


Social Studies, 14.12.2021 15:10



Mathematics, 14.12.2021 15:20

Physics, 14.12.2021 15:20


History, 14.12.2021 15:20

Biology, 14.12.2021 15:20

English, 14.12.2021 15:20

Mathematics, 14.12.2021 15:20


Mathematics, 14.12.2021 15:20



Mathematics, 14.12.2021 15:20

History, 14.12.2021 15:20

English, 14.12.2021 15:20

Biology, 14.12.2021 15:20

Mathematics, 14.12.2021 15:20

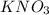 is a neutral salt
is a neutral salt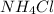 is an acidic salt
is an acidic salt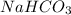 is a basic salt
is a basic salt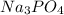 is a neutral salt
is a neutral salt