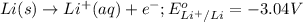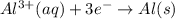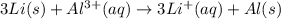Chemistry, 17.06.2021 23:30 kkingstone1231
Using the following portion of the activity series for oxidation half-reactions, determine which combination of reactants will result in a reaction. Li(s) Al3 (aq) eCr(s) Al3 (aq) 3e75) A) Li(s) with Al(s) B) Li(s) with Al3 (aq) C) Li (aq) with Al(s) D) Li (aq) with Al3 (aq)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Using the following portion of the activity series for oxidation half-reactions, determine which com...
Questions

History, 20.12.2021 14:00

English, 20.12.2021 14:00

Mathematics, 20.12.2021 14:00

History, 20.12.2021 14:00



Mathematics, 20.12.2021 14:00

Mathematics, 20.12.2021 14:00


History, 20.12.2021 14:00

Mathematics, 20.12.2021 14:00

Mathematics, 20.12.2021 14:00


Computers and Technology, 20.12.2021 14:00

English, 20.12.2021 14:00

Mathematics, 20.12.2021 14:00




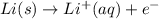
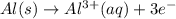
 (aq)
(aq) 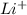 (aq) with Al(s)
(aq) with Al(s)