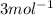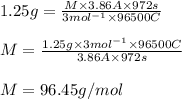Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
A metal forms the fluoride MF3. Electrolysis of the molten fluoride by a current of 3.86 A for 16.2...
Questions

Mathematics, 27.01.2021 19:00

Mathematics, 27.01.2021 19:00

Chemistry, 27.01.2021 19:00



Chemistry, 27.01.2021 19:00

History, 27.01.2021 19:00

English, 27.01.2021 19:00


History, 27.01.2021 19:00





Social Studies, 27.01.2021 19:00

History, 27.01.2021 19:00



Biology, 27.01.2021 19:10

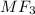
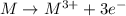
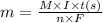 ......(1)
......(1)