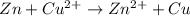Chemistry, 17.06.2021 18:00 michelle7511
Which ionic equation describes a redox reaction? A. Ag(+) + Cl- = AgCl B. 2H(+) + CO3(2-) = CO2 + H2O C. H(+) + OH(-) = H2O D. Zn + Cu(2+) = Zn(2+) + Cu The marking scheme of the past paper this question is from says that the answer is D but how?

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3

Chemistry, 23.06.2019 10:50
Which compound should undergo substitution of the bromine by phenolate anion? draw the structure of the organic product?
Answers: 1

Chemistry, 23.06.2019 16:50
Question 2 (5 points) which of the following materials is useful for making molds because it has a low melting point? question 2 options: wood metal wax sand
Answers: 2
You know the right answer?
Which ionic equation describes a redox reaction? A. Ag(+) + Cl- = AgCl B. 2H(+) + CO3(2-) = CO2 + H2...
Questions

Physics, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00


World Languages, 17.04.2021 14:00


History, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00


Computers and Technology, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00

Mathematics, 17.04.2021 14:00


Mathematics, 17.04.2021 14:00



Biology, 17.04.2021 14:00

History, 17.04.2021 14:10

Biology, 17.04.2021 14:10