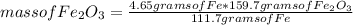Chemistry, 17.06.2021 09:40 tynitenaire
The reaction of iron (III) oxide with carbon monoxide produces iron and carbon dioxide.
Fe2O3(s) + 3C0(9) - 2Fe(s) + 3CO (9)
How many grams of Fe2O3 are required to produce 4.65g Fe? You must show your work to receive full credit.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
The reaction of iron (III) oxide with carbon monoxide produces iron and carbon dioxide.
Fe2O3(s) +...
Questions




English, 04.04.2020 13:50











Mathematics, 04.04.2020 13:51


English, 04.04.2020 13:51