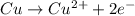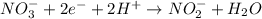What is the final, balanced equation that is formed by combining these two half reactions?
CO
Cu-
> Cu2+
+ 2e-
NO3 + 2e + 2H
>NO2+H20
o Cu2+ + NO3 + + 4e + 2H+ -> Cu + NO3 + H20
o Cu + NO3 + 2H*
>Cu2+ + NO3 + H20
o 2Cu + NO3 + 2H+ —>2Cu?* + NO3 + H20
O Cu+ NO3 + 2H+—
+ NO2 + 2H20

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3


Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1
You know the right answer?
What is the final, balanced equation that is formed by combining these two half reactions?
CO
...
...
Questions

Mathematics, 23.11.2021 14:00




Social Studies, 23.11.2021 14:00

Social Studies, 23.11.2021 14:00

Mathematics, 23.11.2021 14:00





Mathematics, 23.11.2021 14:00

Mathematics, 23.11.2021 14:00

Mathematics, 23.11.2021 14:00

Physics, 23.11.2021 14:00

Business, 23.11.2021 14:00


Social Studies, 23.11.2021 14:00

History, 23.11.2021 14:00

Social Studies, 23.11.2021 14:00

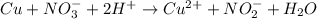 .
.