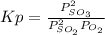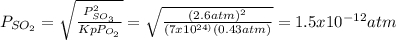Consider the following reaction at 298 K.
2 SO2(g) + O2(g) → 2 SO3(g)
An equilibrium mixture...

Chemistry, 14.06.2021 19:40 hihudgins902
Consider the following reaction at 298 K.
2 SO2(g) + O2(g) → 2 SO3(g)
An equilibrium mixture contains O2(g) and SO3(g) at partial pressures of 0.43 atm and 2.6 atm, respectively. Using data from Appendix 4, determine the equilibrium partial pressure of SO2 in the mixture.
atm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
Questions

Advanced Placement (AP), 26.02.2020 01:49

Mathematics, 26.02.2020 01:49

Computers and Technology, 26.02.2020 01:49




History, 26.02.2020 01:49








English, 26.02.2020 01:49




History, 26.02.2020 01:50