Consider a perfectly insulated and sealed container. Determine the minimum volume in L of the container such that 0.37 L of water will completely evaporate at 25oC. The heat of vaporization of water is 42.68 kJ/mol, and the density of water is 1.00 g/mL. Report your answer to 2 decimal places. g

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
Consider a perfectly insulated and sealed container. Determine the minimum volume in L of the contai...
Questions

Mathematics, 19.03.2021 20:30




Mathematics, 19.03.2021 20:30




Mathematics, 19.03.2021 20:30


SAT, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30

Computers and Technology, 19.03.2021 20:30

History, 19.03.2021 20:30


Chemistry, 19.03.2021 20:30


Mathematics, 19.03.2021 20:30


Chemistry, 19.03.2021 20:30

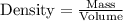 ......(1)
......(1)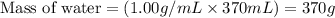
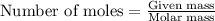 ......(2)
......(2)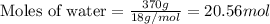
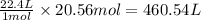 of volume.
of volume.