Chemistry, 10.06.2021 21:50 Imamdiallo18
Given the equation 2Si(s) + 2Cl2(g) --> 2SiCl2(g) + 687 kJ, how much heat is produced when 106 g of Cl2 react?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3
You know the right answer?
Given the equation 2Si(s) + 2Cl2(g) --> 2SiCl2(g) + 687 kJ, how much heat is produced when 106 g...
Questions





Physics, 11.10.2020 23:01




Social Studies, 11.10.2020 23:01






Mathematics, 11.10.2020 23:01


Mathematics, 11.10.2020 23:01



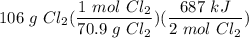 [DA] Divide/Multiply [Cancel out units]:
[DA] Divide/Multiply [Cancel out units]: