
For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equilibrium constant expression is:
a. K = [ H+] [NO2-] / [HNO2]
b. K = [ H+] [N] [O]2 / [HNO2]
c. K = [ H+] [NO2-] / [HNO2]
d. K = [H+]2 [NO2-] / [HNO2]
e. None of these

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1


Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equi...
Questions


Computers and Technology, 11.01.2021 23:00




English, 11.01.2021 23:00

Mathematics, 11.01.2021 23:00


Mathematics, 11.01.2021 23:00

Mathematics, 11.01.2021 23:00

Mathematics, 11.01.2021 23:00

Mathematics, 11.01.2021 23:00

Health, 11.01.2021 23:00

Mathematics, 11.01.2021 23:00

Physics, 11.01.2021 23:00


Biology, 11.01.2021 23:00

Biology, 11.01.2021 23:00


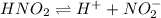
![K = \frac{[H^{+}][NO^{-}_{2}]}{[HNO_{2}]}](/tpl/images/1365/7220/b80db.png)


