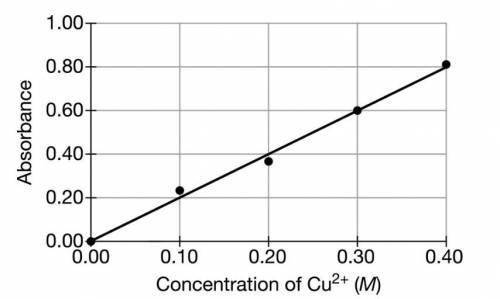
A student is given a sample of CuSO4(s) that contains a solid impurity that is soluble and colorless. The student wants to determine the amount of CuSO4 in the sample and decides to use a spectrophotometer. First, the student prepares a calibration graph by measuring the absorbances of CuSO4(aq) solutions of known concentrations. The graph is shown below.
(a) The student dissolves the entire impure sample of CuSO4(s) in enough distilled water to make 100.mL of solution. Then the student measures the absorbance of the solution and observes that it is 0.30. Determine the concentration of CuSO4(aq) in the solution.
(b) Calculate the number of moles of CuSO4 that were in the impure sample of CuSO4(s).
(c) In addition to the number of moles of CuSO4 calculated in part (b), what other quantity must be measured in order to calculate the mass percentage of CuSO4 in the impure sample of CuSO4(s)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
A student is given a sample of CuSO4(s) that contains a solid impurity that is soluble and colorless...
Questions

Mathematics, 08.11.2020 19:20


Arts, 08.11.2020 19:20









English, 08.11.2020 19:20

Mathematics, 08.11.2020 19:20

Chemistry, 08.11.2020 19:20

Social Studies, 08.11.2020 19:20

History, 08.11.2020 19:20


Biology, 08.11.2020 19:20

Mathematics, 08.11.2020 19:20

Mathematics, 08.11.2020 19:20