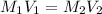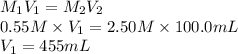Chemistry, 03.06.2021 23:50 animerocks07
Q Q 3. (08.02 MC)
What volume of 0.550 M KBr solution can you make from 100.0 mL of 2.50 M KBr? (3 points)
O 455 mL
576 ml
924 ml
O 1.230 ml

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
Q Q 3. (08.02 MC)
What volume of 0.550 M KBr solution can you make from 100.0 mL of 2.50 M KBr? (3...
Questions






Chemistry, 02.10.2019 20:20








Mathematics, 02.10.2019 20:20



History, 02.10.2019 20:20

Biology, 02.10.2019 20:20


History, 02.10.2019 20:20

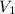 = ?,
= ?, 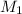 = 0.55 M
= 0.55 M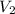 = 100.0 mL,
= 100.0 mL, 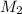 = 2.50 M
= 2.50 M