
Chemistry, 03.06.2021 18:00 hjeffrey168
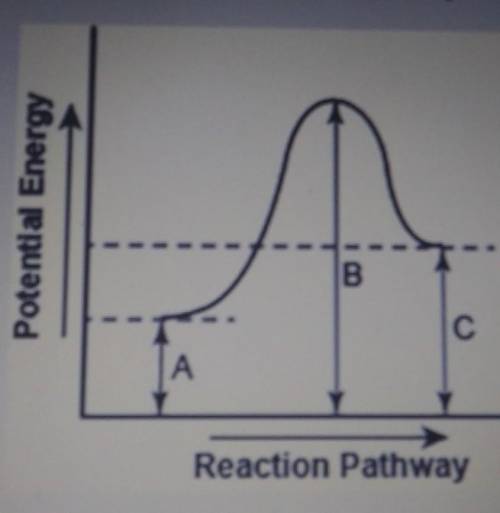
The diagram below shows the potential energy changes for a reaction pathway.
Part 1: Does the diagram illustrate an enothermic or an exothermic reaction? Give reasons to support your answer.
Part 2: Describe how you can determine the total change in ethalpy and activation energy from the diagram and if each is positive or negative.
Brainliest if you give the definition of endo and exothermic reactions, and how you know the change in enthalpy even if you put nothing else.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
The diagram below shows the potential energy changes for a reaction pathway.
Part 1: Does the diagr...
Questions

Physics, 16.09.2019 03:50

History, 16.09.2019 03:50


Social Studies, 16.09.2019 03:50





English, 16.09.2019 03:50


Physics, 16.09.2019 03:50


SAT, 16.09.2019 03:50




Physics, 16.09.2019 03:50



Business, 16.09.2019 03:50



