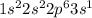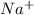Chemistry, 03.06.2021 17:00 jacobiroberts18
Sodium reacts with chlorine to form sodium chloride. Which statements describe what happens to the sodium atoms in this reaction? 1. Sodium atoms form positive ions. 2. Sodium atoms form negative ions. 3. Sodium atoms gain electrons. 4. Sodium atoms lose electrons a. 1 and 3 b. 1 and 4 c. 2 and 3 d. 2 and 4

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
Sodium reacts with chlorine to form sodium chloride. Which statements describe what happens to the s...
Questions

Mathematics, 06.12.2021 23:00


Mathematics, 06.12.2021 23:00



Mathematics, 06.12.2021 23:00

Spanish, 06.12.2021 23:00


English, 06.12.2021 23:00


Geography, 06.12.2021 23:00

Mathematics, 06.12.2021 23:00

Mathematics, 06.12.2021 23:00


Mathematics, 06.12.2021 23:00

Mathematics, 06.12.2021 23:00

Mathematics, 06.12.2021 23:00

Mathematics, 06.12.2021 23:00