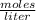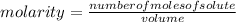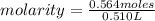Chemistry, 03.06.2021 07:20 yselahernandez02
a chemist dissolves 0.564 moles of manganese (IV) oxide (MnO2) in water, and adds enough water to make 0.510 L of solution. Calculate the molarity of the solution.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
a chemist dissolves 0.564 moles of manganese (IV) oxide (MnO2) in water, and adds enough water to ma...
Questions




Mathematics, 29.07.2021 19:00

Mathematics, 29.07.2021 19:00

Mathematics, 29.07.2021 19:00


English, 29.07.2021 19:00



Mathematics, 29.07.2021 19:00


Mathematics, 29.07.2021 19:00





Social Studies, 29.07.2021 19:10


English, 29.07.2021 19:10