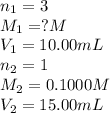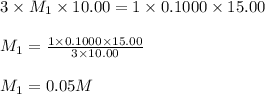Chemistry, 03.06.2021 04:30 elissiashontelbrown
g 10.00 mL of phosphoric acid (H3PO4) are titrated with 0.1000 M sodium hydroxide. 15.00 mL of the sodium hydroxide solution are used in this experiment. Determine the molarity of phosphoric acid.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1


Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
g 10.00 mL of phosphoric acid (H3PO4) are titrated with 0.1000 M sodium hydroxide. 15.00 mL of the s...
Questions

Mathematics, 15.04.2020 20:28


History, 15.04.2020 20:28







History, 15.04.2020 20:28


Computers and Technology, 15.04.2020 20:28

Geography, 15.04.2020 20:29





Mathematics, 15.04.2020 20:30


Mathematics, 15.04.2020 20:30

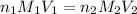 ........(1)
........(1)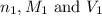 are the n-factor, molarity and volume of acid that is
are the n-factor, molarity and volume of acid that is 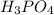
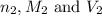 are the n-factor, molarity and volume of the base that is NaOH
are the n-factor, molarity and volume of the base that is NaOH