
Chemistry, 01.06.2021 19:00 okokalyssa
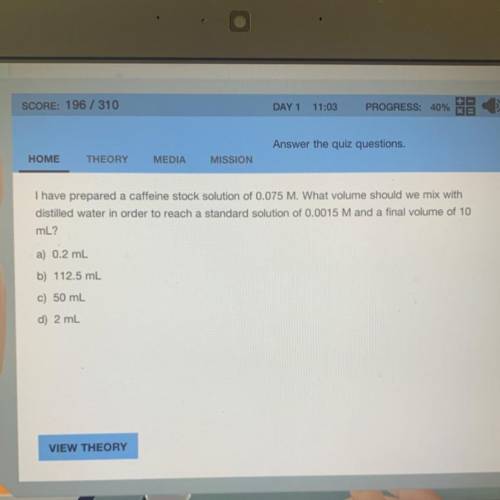
I have prepared a caffeine stock solution of 0.075 M. What volume should we mix with
distilled water in order to reach a standard solution of 0.0015 M and a final volume of 10
mL?
a) 0.2 mL
b) 112.5 mL
c) 50 mL
d) 2 mL
PLEASE HELP!
No Links!
I will award brainliest to the first person to answer

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


You know the right answer?
I have prepared a caffeine stock solution of 0.075 M. What volume should we mix with
distilled wate...
Questions


Mathematics, 08.09.2021 04:50

Mathematics, 08.09.2021 04:50



Chemistry, 08.09.2021 04:50





Social Studies, 08.09.2021 04:50

Social Studies, 08.09.2021 04:50

History, 08.09.2021 04:50


Mathematics, 08.09.2021 04:50

Mathematics, 08.09.2021 04:50

Mathematics, 08.09.2021 04:50

Mathematics, 08.09.2021 04:50

English, 08.09.2021 04:50

Mathematics, 08.09.2021 04:50



