
An engine operates on a Carnot cycle that uses 1mole of an ideal gas as the
working substance and operates from a most compressed stage of 100 Nm and
327 K. It expands isothermally to a pressure of 90 Nm and then adiabatically to a
most expanded stage of 27 K. Calculate the AU, 9, and w for each step. Calculate
the net work done and the efficiency of the cycle [Cv, m for the gas) is 25 J/k/mol.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1


Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
You know the right answer?
An engine operates on a Carnot cycle that uses 1mole of an ideal gas as the
working substance and o...
Questions

Chemistry, 26.10.2019 15:43


Mathematics, 26.10.2019 15:43








History, 26.10.2019 15:43

Biology, 26.10.2019 15:43

History, 26.10.2019 15:43



Computers and Technology, 26.10.2019 15:43


Chemistry, 26.10.2019 15:43


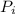 = 100 N·m
= 100 N·m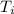 = 327 K
= 327 K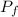 = 90 N·m
= 90 N·m

