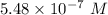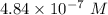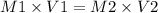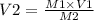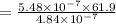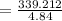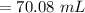A chemist must dilute 61.9 mL of 548. nM aqueous sodium carbonate (Na2CO3) solution until the concentration falls to 484. nM . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Round your answer to 3 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2


Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
A chemist must dilute 61.9 mL of 548. nM aqueous sodium carbonate (Na2CO3) solution until the concen...
Questions

Mathematics, 18.08.2019 22:00



English, 18.08.2019 22:00

Mathematics, 18.08.2019 22:00








Social Studies, 18.08.2019 22:00

Mathematics, 18.08.2019 22:00


Mathematics, 18.08.2019 22:00




History, 18.08.2019 22:00