Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
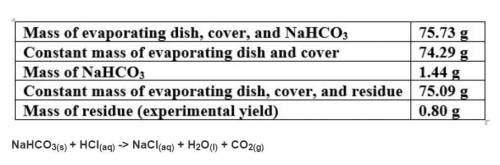
Based on a theoretical yield of 1.00 grams NaCl, calculate the mass in grams of hydrochloric acid yo...
Questions




English, 05.11.2020 01:20




Mathematics, 05.11.2020 01:20

Social Studies, 05.11.2020 01:20

Physics, 05.11.2020 01:20





Mathematics, 05.11.2020 01:20


Mathematics, 05.11.2020 01:20


Mathematics, 05.11.2020 01:20

Mathematics, 05.11.2020 01:20