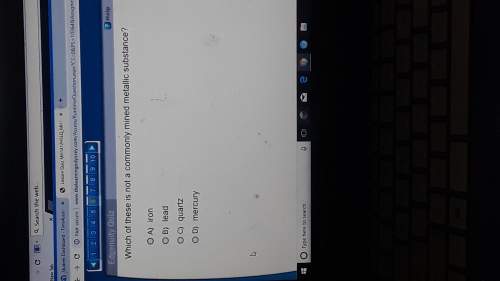
The volume of a fixed amount of gas is doubled, and the absolute temperature is doubled. According to the ideal gas law, how has the pressure of the gas changed? A. It has stayed the same. B. It has decreased to one-half its original value. C. It has increased to four times its original value. D. It has increased to two times its original value.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
The volume of a fixed amount of gas is doubled, and the absolute temperature is doubled. According t...
Questions

Mathematics, 07.02.2022 06:30


Social Studies, 07.02.2022 06:30

Mathematics, 07.02.2022 06:30

Mathematics, 07.02.2022 06:30

Mathematics, 07.02.2022 06:30



Biology, 07.02.2022 06:30



Mathematics, 07.02.2022 06:30

Geography, 07.02.2022 06:30



Mathematics, 07.02.2022 06:30

Mathematics, 07.02.2022 06:30

Mathematics, 07.02.2022 06:30