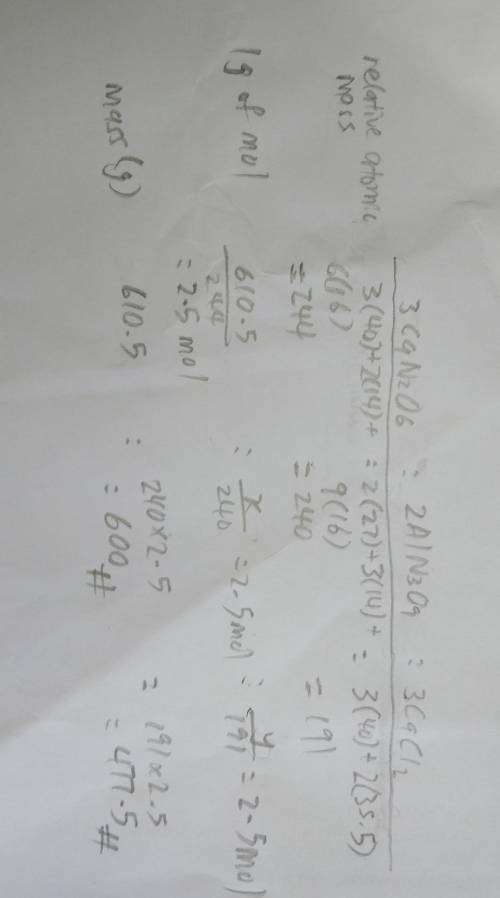Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 23.06.2019 13:00
What did the experiments of scientists after john dalton reveal about his atomic theory?
Answers: 1

Chemistry, 23.06.2019 16:30
There is a set up transformer that doubles the voltage. if the primary coil has a voltage of 10 v
Answers: 2

Chemistry, 23.06.2019 17:10
Two changes are described below. a green banana turns yellow and ripens. a layer of rust forms on an iron nail. which statement is true about the two changes? a) both are chemical changes because new substances are formed. b) both are physical changes because only the physical state of the substances change. c) a is a physical change due to a change of state, but b is a chemical change because new molecules are formed. d) a is a chemical change due to a change of state, but b is a physical change because new molecules are formed.
Answers: 3
You know the right answer?
Consider the following balanced equation: 3Ca(NO3)2 + 2AlCl3 --> 2Al(NO3)3 + 3CaCl2. If 610.5 g o...
Questions



History, 17.04.2020 03:14




Mathematics, 17.04.2020 03:14

Mathematics, 17.04.2020 03:14

History, 17.04.2020 03:14

Mathematics, 17.04.2020 03:14

Advanced Placement (AP), 17.04.2020 03:14



Physics, 17.04.2020 03:14


Mathematics, 17.04.2020 03:14