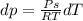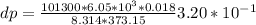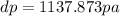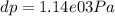Chemistry, 18.05.2021 19:10 chasewilkinson123
Use the fact that to determine how much the pressure must change in order to lower the boiling point of water by a small amount 3.20e-01 K. You may assume that the entropy and density of the liquid and gas are roughly constant for these small changes. You may also assume that the volume per molecule of liquid water is approximately zero compared to that of water vapor, and that water vapor is an ideal gas. Useful constants: Atmospheric pressure is 101300 Pa The boiling point of water at atmospheric pressure is 373.15 K The entropy difference between liquid and gas per kilogram is 6.05e 03 The molecular weight of water is 0.018 kg/mol. (a) 0.00e 00 Pa (b) 1.14e 03 Pa (c) 6.85e 26 Pa (d) 4.24e 05 Pa (e) 3.81e 28 Pa

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3
You know the right answer?
Use the fact that to determine how much the pressure must change in order to lower the boiling point...
Questions



Mathematics, 18.11.2020 18:50


Chemistry, 18.11.2020 18:50





Mathematics, 18.11.2020 18:50

Biology, 18.11.2020 18:50






Social Studies, 18.11.2020 18:50


Social Studies, 18.11.2020 18:50

History, 18.11.2020 18:50

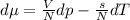 to determine how much the pressure must change in order to lower the boiling point of water by a small amount 3.20e-01 K. You may assume that the entropy and density of the liquid and gas are roughly constant for these small changes. You may also assume that the volume per molecule of liquid water is approximately zero compared to that of water vapor, and that water vapor is an ideal gas. Useful constants: Atmospheric pressure is 101300 Pa The boiling point of water at atmospheric pressure is 373.15 K The entropy difference between liquid and gas per kilogram is 6.05e 03 J/kgK The molecular weight of water is 0.018 kg/mol. (a) 0.00e 00 Pa (b) 1.14e 03 Pa (c) 6.85e 26 Pa (d) 4.24e 05 Pa (e) 3.81e 28 Pa
to determine how much the pressure must change in order to lower the boiling point of water by a small amount 3.20e-01 K. You may assume that the entropy and density of the liquid and gas are roughly constant for these small changes. You may also assume that the volume per molecule of liquid water is approximately zero compared to that of water vapor, and that water vapor is an ideal gas. Useful constants: Atmospheric pressure is 101300 Pa The boiling point of water at atmospheric pressure is 373.15 K The entropy difference between liquid and gas per kilogram is 6.05e 03 J/kgK The molecular weight of water is 0.018 kg/mol. (a) 0.00e 00 Pa (b) 1.14e 03 Pa (c) 6.85e 26 Pa (d) 4.24e 05 Pa (e) 3.81e 28 Pa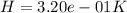
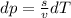
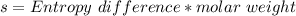
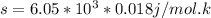
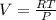 (from ideal gas equation)
(from ideal gas equation)