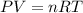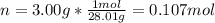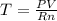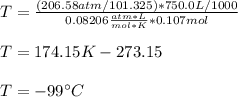Chemistry, 17.05.2021 16:50 colyernicholas44
A sample of nitrogen gas in a 750.0 mL sealed flask exerts a pressure of 206.58 kPa. Calculate the temperature of the gas if the flask contains 3.00 grams of gas.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
A sample of nitrogen gas in a 750.0 mL sealed flask exerts a pressure of 206.58 kPa. Calculate the t...
Questions


Chemistry, 26.04.2021 21:20

English, 26.04.2021 21:20

German, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Physics, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20


Mathematics, 26.04.2021 21:20

Geography, 26.04.2021 21:20


Spanish, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20



Mathematics, 26.04.2021 21:20