
Chemistry, 14.05.2021 06:20 butterflyrhodes01
Considere la combustión del monóxido de carbono (CO) en oxígeno gaseoso: 2CO(g) + O2(g) → 2CO2(g) Si la reacción se inicia con 3.60 moles de CO, calcule el número de moles de CO2 que se producen si hay suficiente oxígeno para reaccionar con todo el CO.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Considere la combustión del monóxido de carbono (CO) en oxígeno gaseoso: 2CO(g) + O2(g) → 2CO2(g) S...
Questions



Mathematics, 07.01.2021 17:40




English, 07.01.2021 17:40

Mathematics, 07.01.2021 17:40


Spanish, 07.01.2021 17:40


Mathematics, 07.01.2021 17:40

Spanish, 07.01.2021 17:40

History, 07.01.2021 17:40

Physics, 07.01.2021 17:40

Social Studies, 07.01.2021 17:40

Mathematics, 07.01.2021 17:40



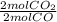 = 3.60 moles CO₂
= 3.60 moles CO₂

