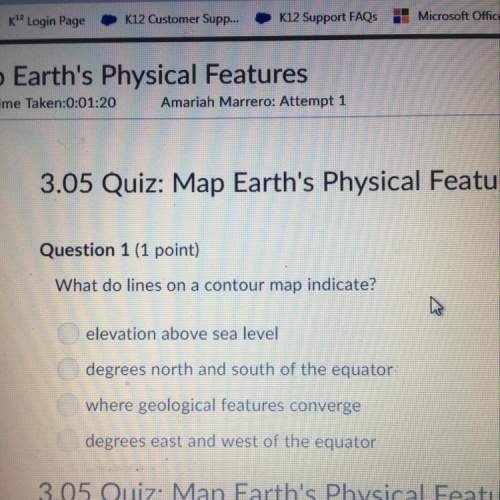
PLEASE PLEASE HELP WILL MARK BRAINLIEST
(07.04 HC)
A 72.0-gram piece of metal at 96.0...

Chemistry, 07.05.2021 07:10 julianastri6841
PLEASE PLEASE HELP WILL MARK BRAINLIEST
(07.04 HC)
A 72.0-gram piece of metal at 96.0 °C is placed in 130.0 g of water in a calorimeter at 25.5 °C. The final temperature in the calorimeter is 31.0 °C. Determine the specific heat of the metal. Show your work by listing various steps, and explain how the law of conservation of energy applies to this situation.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1


Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Questions



Biology, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01



Mathematics, 20.09.2020 14:01

Computers and Technology, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01

Chemistry, 20.09.2020 14:01


History, 20.09.2020 14:01


Advanced Placement (AP), 20.09.2020 14:01

Mathematics, 20.09.2020 14:01

Biology, 20.09.2020 14:01