Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1

Chemistry, 23.06.2019 16:50
An element x has two isotopes. x–15 has 7 protons and 8 neutrons. determine the atomic number and mass number of the second isotope, which has 10 neutrons in its nucleus.
Answers: 1
You know the right answer?
10 points. Please help.
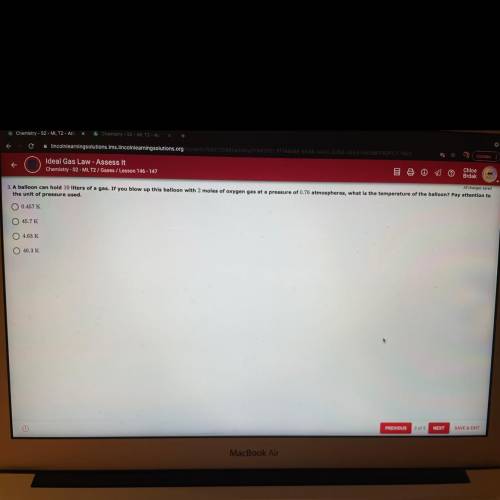
A balloon can hold 10 liters of a gas. If you blow up this balloon with 2...
Questions


English, 23.07.2021 14:00


Social Studies, 23.07.2021 14:00




Mathematics, 23.07.2021 14:00

English, 23.07.2021 14:00

Biology, 23.07.2021 14:00


Biology, 23.07.2021 14:00

Mathematics, 23.07.2021 14:00

Chemistry, 23.07.2021 14:00

Mathematics, 23.07.2021 14:00



Biology, 23.07.2021 14:00