PLEASEEE HELP A chemist mixed two substances
together: a blue powder with no smell
and a col...

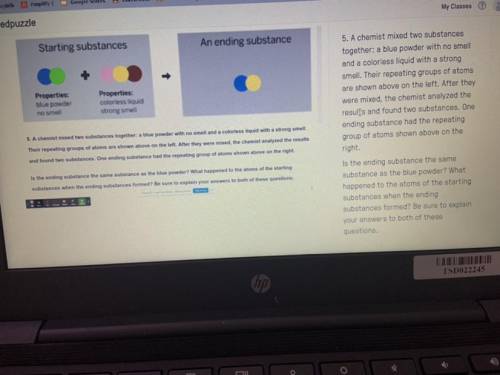
PLEASEEE HELP A chemist mixed two substances
together: a blue powder with no smell
and a colorless liquid with a strong
smell. Their repeating groups of atoms
are shown above on the left. After they
were mixed, the chemist analyzed the
results and found two substances. One
ending substance had the repeating
group of atoms shown above on the
right.
Is the ending substance the same
substance as the blue powder? What
happened to the atoms of the starting
substances when the ending
substances formed? Be sure to explain
your answers to both of these

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
Questions

Mathematics, 29.03.2021 22:30


Mathematics, 29.03.2021 22:30

Health, 29.03.2021 22:30







Social Studies, 29.03.2021 22:30



Mathematics, 29.03.2021 22:30

English, 29.03.2021 22:30







