Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 04:00
Actual ingredients of lab (the cookies i am actually making) 1/2 cup sugar 1/2 cup brown sugar 1 1/3 stick margarine 1 egg 1/2 tsp salt 1 tsp vanilla 1/2 tsp baking soda 1 1/2 cup flour 1 1/3 cup chocolate chip can you answer the questions below ? discussion 1. suppose you are given the following amounts of ingredients: 1 dozen eggs 24 tsp. of vanilla 1 lb. (82 tsp.) of salt 1 lb. (84 tsp.) of baking soda 3 cups of chocolate chips 5 lb. (11 cups) of sugar 2 lb. (4 cups) of brown sugar 1 lb. (4 sticks) of margarine a. for each ingredient, calculate how many cookies could be prepared if all of that ingredient were consumed. (for example, the recipe shows that using 1 egg- with the right amounts of the other ingredients- yields 24 cookies. how many cookies can you make if the recipe is increased proportionately for 12 eggs? ) b. to determine the limiting reactant for the new ingredients list, identify which ingredient will result in the fewest number of cookies. c. what is the maximum number of cookies that can be produced from the new amounts of ingredients?
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2
You know the right answer?
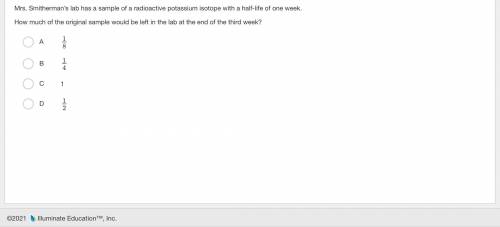
Question is in picture
...
...
Questions

Mathematics, 05.02.2021 04:30

Mathematics, 05.02.2021 04:30


Arts, 05.02.2021 04:30

Mathematics, 05.02.2021 04:30

History, 05.02.2021 04:30

Social Studies, 05.02.2021 04:30


Mathematics, 05.02.2021 04:30

Geography, 05.02.2021 04:30

English, 05.02.2021 04:30

Mathematics, 05.02.2021 04:30

Mathematics, 05.02.2021 04:30

Mathematics, 05.02.2021 04:30





Mathematics, 05.02.2021 04:30

Mathematics, 05.02.2021 04:30