Chemistry, 19.12.2019 13:31 dakshshberry
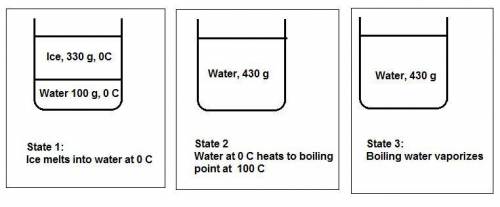
Water's heat of fusion is 80. cal/g , its specific heat is 1.0calg⋅∘c, and its heat of vaporization is 540 cal/g . a canister is filled with 330 g of ice and 100. g of liquid water, both at 0 ∘c . the canister is placed in an oven until all the h2o has boiled off and the canister is empty. how much energy in calories was absorbed?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Water's heat of fusion is 80. cal/g , its specific heat is 1.0calg⋅∘c, and its heat of vaporization...
Questions

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Arts, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

English, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

History, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Chemistry, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01