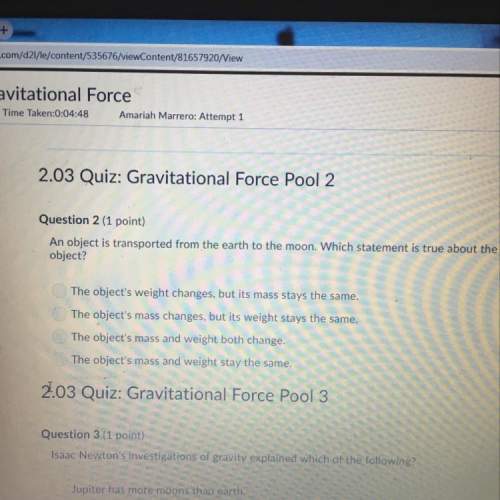
Chemistry, 23.09.2019 15:00 cgkiepe5759
He first-order reaction, so2cl2 → so2 + cl2, has a half-life of 8.75 hours at 593 k. how long will it take for the concentration of so2cl2 to fall to 16.5% of its initial value? the first-order reaction, so2cl2 → so2 + cl2, has a half-life of 8.75 hours at 593 k. how long will it take for the concentration of so2cl2 to fall to 16.5% of its initial value? 22.7 hr 0.143 hr 2.28 hr 6.99 hr

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
He first-order reaction, so2cl2 → so2 + cl2, has a half-life of 8.75 hours at 593 k. how long will i...
Questions

Mathematics, 03.02.2020 02:55

Mathematics, 03.02.2020 02:55


Social Studies, 03.02.2020 02:55

Mathematics, 03.02.2020 02:55









Biology, 03.02.2020 02:55


Computers and Technology, 03.02.2020 02:56



Advanced Placement (AP), 03.02.2020 02:56

Mathematics, 03.02.2020 02:56