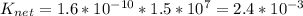The solubility of silver chloride can be increased by dissolving it in a solution containing ammonia. agcl (s) ag+ (aq) + cl- (aq) k1 = 1.6 x 10-10 ag+ (aq) + 2nh3 (aq) ag(nh3)2+ (aq) k2 = 1.5 x 107 what is the value of the equilibrium constant for the overall reaction? agcl (s) + 2nh3 (aq) ag(nh3)2+ (aq) + cl- (aq) knet = ?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1
You know the right answer?
The solubility of silver chloride can be increased by dissolving it in a solution containing ammonia...
Questions


Chemistry, 04.06.2020 21:04



Social Studies, 04.06.2020 21:04

Mathematics, 04.06.2020 21:04


Biology, 04.06.2020 21:04

Mathematics, 04.06.2020 21:04

Mathematics, 04.06.2020 21:04

Mathematics, 04.06.2020 21:04

Biology, 04.06.2020 21:04







World Languages, 04.06.2020 21:04

![K_{net}=[Cl^-]*[Ag(NH_3)_2^{+2}]=2.4*10^{-3}](/tpl/images/0309/4158/aeebe.png)
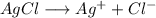
![K_1=[Ag^+]*[Cl^-]](/tpl/images/0309/4158/2f3df.png)

![K_2=\frac{[Ag(NH_3)_2^{+2}]}{[Ag^+]}](/tpl/images/0309/4158/d8bb8.png)
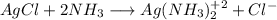
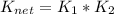
![K_{net}=[Ag^+]*[Cl^-]*\frac{[Ag(NH_3)_2^{+2}]}{[Ag^+]}](/tpl/images/0309/4158/ccfbe.png)
![K_{net}=[Cl^-]*[Ag(NH_3)_2^{+2}]](/tpl/images/0309/4158/2d974.png)