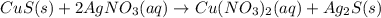Chemistry, 15.11.2019 20:31 Tringirl233
E. solid copper sulfide and silver nitrate react to form copper (ii) nitrate and solid silver sulfide. write a balanced chemical equation that describes the reaction. identify the oxidation number of each element in the reaction. (you do not need to include the total contribution of charge.) is this reaction a redox reaction or a non-redox reaction? explain your answer.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1


Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
E. solid copper sulfide and silver nitrate react to form copper (ii) nitrate and solid silver sulfid...
Questions

Social Studies, 20.10.2019 14:30


Mathematics, 20.10.2019 14:30


Mathematics, 20.10.2019 14:30

Health, 20.10.2019 14:30



Geography, 20.10.2019 14:30



Spanish, 20.10.2019 14:30





Mathematics, 20.10.2019 14:30

Mathematics, 20.10.2019 14:30


English, 20.10.2019 14:30