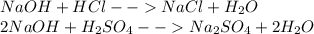Chemistry, 28.08.2019 21:30 michaelchavez6959127
You have separate solutions of hcl and h2so4 with the same concentrations in terms of molarity. you wish to neutralize a solution of naoh. which acid solution would require more volume (in ml) to neutralize the base?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
You have separate solutions of hcl and h2so4 with the same concentrations in terms of molarity. you...
Questions





Mathematics, 15.12.2020 02:00



Biology, 15.12.2020 02:00



Mathematics, 15.12.2020 02:00


Biology, 15.12.2020 02:00