
Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
How do you calculate experimental mole ratio?...
Questions

Mathematics, 05.11.2020 01:20


Mathematics, 05.11.2020 01:20

Mathematics, 05.11.2020 01:20


English, 05.11.2020 01:20

Mathematics, 05.11.2020 01:20


Mathematics, 05.11.2020 01:20

French, 05.11.2020 01:20



English, 05.11.2020 01:20


Health, 05.11.2020 01:20



Mathematics, 05.11.2020 01:20




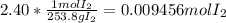
 =
= 


