
Chemistry, 17.10.2019 03:00 Foxfire5109
How many liters of methane gas (ch4) need to be combusted to produce 8.5 liters of water vapor, if all measurements are taken at the same temperature and pressure? show all of the work used to solve this problem. ch4 (g) + 2o2 (g) yields co2 (g) + 2h2o (g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
You know the right answer?
How many liters of methane gas (ch4) need to be combusted to produce 8.5 liters of water vapor, if a...
Questions


Mathematics, 14.09.2021 09:30

Mathematics, 14.09.2021 09:30


Chemistry, 14.09.2021 09:30


Chemistry, 14.09.2021 09:30


Mathematics, 14.09.2021 09:30

Mathematics, 14.09.2021 09:30

Biology, 14.09.2021 09:30


English, 14.09.2021 09:30






Mathematics, 14.09.2021 09:30

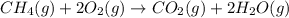
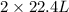 volume of water vapor produced from 22.4 L volume of methane gas
volume of water vapor produced from 22.4 L volume of methane gas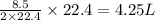 volume of methane gas
volume of methane gas

