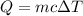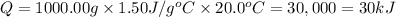Chemistry, 02.09.2019 02:20 izzyisawesome5232
Asample of octane (c8h18 that has a mass of 0.750 g is burned in a bomb calorimeter. as a result, the temperature of the calorimeter increases from 21.0°c to 41.0°c. the specific heat of the calorimeter is 1.50 j/(g °c, and its mass is 1.00 kg. how much heat is released during the combustion of this sample? use .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
Asample of octane (c8h18 that has a mass of 0.750 g is burned in a bomb calorimeter. as a result, th...
Questions

History, 17.09.2019 15:10

English, 17.09.2019 15:10

Physics, 17.09.2019 15:10


Social Studies, 17.09.2019 15:10


Mathematics, 17.09.2019 15:10

Mathematics, 17.09.2019 15:10

Biology, 17.09.2019 15:10




Physics, 17.09.2019 15:10


Biology, 17.09.2019 15:10

English, 17.09.2019 15:10


Mathematics, 17.09.2019 15:10


Health, 17.09.2019 15:10