
Chemistry, 17.10.2019 20:30 maskythegamer
How many grams of each of the following substances will dissolve in 100 ml of cold water; ce(io3)4, raso4, (nh4)2seo4?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
How many grams of each of the following substances will dissolve in 100 ml of cold water; ce(io3)4,...
Questions

Biology, 28.08.2019 04:30



Social Studies, 28.08.2019 04:30

Mathematics, 28.08.2019 04:30


Mathematics, 28.08.2019 04:30

History, 28.08.2019 04:30


History, 28.08.2019 04:30

History, 28.08.2019 04:30

Mathematics, 28.08.2019 04:30

Mathematics, 28.08.2019 04:30





Chemistry, 28.08.2019 04:30


Physics, 28.08.2019 04:30

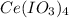 = 0.123g ,
= 0.123g , 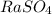 =
= 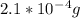 and
and 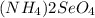 = 96 g
= 96 g 

