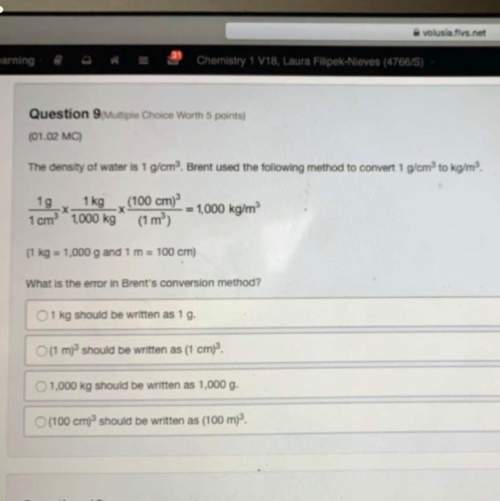
Ireally really need . i'll fan and medal. ! 40 points! and show all the work.
heat o...

Chemistry, 08.10.2019 07:30 paytonxxburns05
Ireally really need . i'll fan and medal. ! 40 points! and show all the work.
heat of combustion lab directions: in this activity, you will use experimental data obtained from a bomb calorimeter to calculate the molar heat of methane, ch4 a hydrocarbon. you will then compare the experimental results with the actual molar heat of combustion of methane and calculate the percent error.
1. you may recall that the products of the complete combustion of a hydrocarbon are water vapor and carbon dioxide gas. write the balanced equation showing the combustion of methane. do not forget to include the states of matter of the reactants and the products. hint: methane is a gas at standard temperature and pressure.
to begin the experiment, 1.11g of methane ch4 is burned in a bomb calorimeter containing 1000 grams of water. the initial temperature of water is 24.85o c. the specific heat of water is 4.184 j/g oc. the heat capacity of the calorimeter is 695 j/ oc . after the reaction the final temperature of the water is 35.65o c.
2. calculate the change in temperature, δt
3. using the formula water q =m•c• δt ,calculate the heat absorbed by the water
4. using the formula cal cal q =c • δt, calculate the heat absorbed by the calorimeter.
5. the total heat absorbed by the water and the calorimeter can be calculated by adding the heat calculated in steps 3 and 4. the amount of heat released by the reaction is equal to the amount of heat absorbed with the negative sign as this is an exothermic reaction. using the formula ∆h = -(qcal qwater + ) , calculate the total heat of combustion.
6. evaluate the information contained in this calculation and complete the following sentence: this calculation shows that burning grams of methane [takes in/gives off] energy.
7. the molar mass of methane is 16.04 g/mol. calculate the number of moles of methane burned in the experiment.
8. what is the experimental molar heat of combustion?
9. the accepted value for the heat of combustion of methane is -890 kj/mol. explain why the experimental data might differ from the theoretical value.
10.give the formula % error = (theoretical value - experimental value divided by the theoretical value) times 100. , calculate the percent error for the experiment.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3


Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
Questions


World Languages, 18.12.2020 02:50


Mathematics, 18.12.2020 02:50

Biology, 18.12.2020 02:50


History, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50





Mathematics, 18.12.2020 02:50

Social Studies, 18.12.2020 02:50


Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50