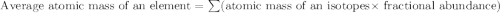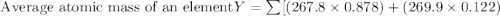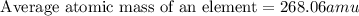Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Anewly discovered element, y, has two naturally occurring isotopes. 87.8 percent of the sample is an...
Questions





English, 26.08.2020 05:01


Mathematics, 26.08.2020 05:01

Mathematics, 26.08.2020 05:01

Mathematics, 26.08.2020 05:01

Mathematics, 26.08.2020 05:01

Biology, 26.08.2020 05:01


Computers and Technology, 26.08.2020 05:01

Chemistry, 26.08.2020 05:01

History, 26.08.2020 05:01





Computers and Technology, 26.08.2020 05:01