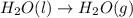Evaporating water is an example of a physical change because (2 points) question 7 options: 1) bonds are broken and atoms are rearranged. 2) the atoms are pulled apart to form a new substance. 3) it does not involve intermolecular forces or particle motion. 4) the identity of the substance does not change.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
Evaporating water is an example of a physical change because (2 points) question 7 options: 1) bond...
Questions


Mathematics, 08.07.2019 13:30



Mathematics, 08.07.2019 13:30

Mathematics, 08.07.2019 13:30



History, 08.07.2019 13:30



Mathematics, 08.07.2019 13:30

English, 08.07.2019 13:30



Mathematics, 08.07.2019 13:30



English, 08.07.2019 13:30