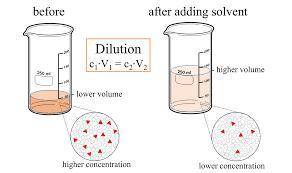
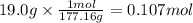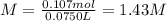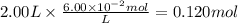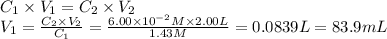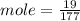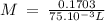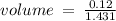On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by adding 19.0g of luminol into a total volume of 75.0ml of h2o.
a)what is the molarity of the stock solution of luminol?
anwer i got: molarity of luminol solution = 1.43m b)before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00×10−2 m. the diluted solution is then placed in a spray bottle for application on the desired surfaces.
i cannot get the correct answer for "c" have tried: 172ml,11.9ml, and 1.19*10^4. the only other possibility that i can come up with is: 83.9ml. would this one be i still completely out to
c)how many moles of luminol are present in 2.00 l of the diluted spray?
anwer i got: moles of luminol = 0.120mol what volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)?
express your answer in milliliters.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
On part "c": the forensic technician at a crime scene has just prepared a luminol stock solution by...
Questions


Mathematics, 01.07.2019 08:30

Mathematics, 01.07.2019 08:30

Mathematics, 01.07.2019 08:30

Mathematics, 01.07.2019 08:30

Mathematics, 01.07.2019 08:30



Social Studies, 01.07.2019 08:30


Chemistry, 01.07.2019 08:30




Computers and Technology, 01.07.2019 08:30


History, 01.07.2019 08:30



Mathematics, 01.07.2019 08:30