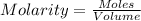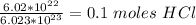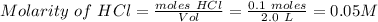Chemistry, 10.11.2019 10:31 ddmoorehouseov75lc
Calculate the molarity of the solution: 6.02x10^22 molecules of hcl (molecular weight = 36.5 g/mole) in 2.0 liters of water.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Calculate the molarity of the solution: 6.02x10^22 molecules of hcl (molecular weight = 36.5 g/mole...
Questions