
Chemistry, 29.09.2019 13:10 Kingmoney959
According to hess’s law, if a series of intermediate reactions are combined, the enthalpy change of the overall reaction is
the difference between the enthalpies of the intermediate reactions.
the sum of the enthalpy changes of the intermediate reactions.
the product of the enthalpy changes of the intermediate reactions.
the fraction of the individual enthalpies of the intermediate reactions.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

You know the right answer?
According to hess’s law, if a series of intermediate reactions are combined, the enthalpy change of...
Questions



Mathematics, 04.02.2021 01:00


English, 04.02.2021 01:00

Mathematics, 04.02.2021 01:00


Chemistry, 04.02.2021 01:00










Mathematics, 04.02.2021 01:00


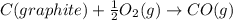
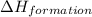
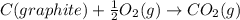
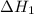
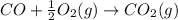
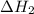
![\Delta H_{formation}=[n\times \Delta H_1]+[n\times \Delta H_2]](/tpl/images/0273/8162/6e820.png)



