
Chemistry, 07.10.2019 08:02 ilovejustinbieber42
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the solubility product constant of cu3(aso4)2.
9.1 x 10^-4 m
3.4 x 10^-2 m
3.7 x 10^-8 m
8.7 x 10^-2 m

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the...
Questions

Mathematics, 11.04.2020 06:36

Mathematics, 11.04.2020 06:36

English, 11.04.2020 06:36


Spanish, 11.04.2020 06:36


Mathematics, 11.04.2020 06:36

English, 11.04.2020 06:36


English, 11.04.2020 06:36


English, 11.04.2020 06:36



Physics, 11.04.2020 06:36

Biology, 11.04.2020 06:36


English, 11.04.2020 06:37


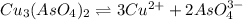
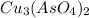 will be given by:
will be given by: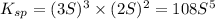
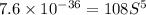
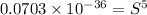
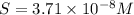
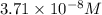 .
.

