Ammonia is produced by the following reaction.
3h2(g) + n2(g) > 2nh3(g)
...

Chemistry, 14.10.2019 22:00 yeicooyola3
Ammonia is produced by the following reaction.
3h2(g) + n2(g) > 2nh3(g)
when 7.00 g of hydrogen react with 70.0 g of nitrogen, hydrogen is considered the limiting reactant because
7.5 mol of hydrogen would be needed to consume the available nitrogen.
7.5 mol of nitrogen would be needed to consume the available hydrogen.
hydrogen would produce 7.5 mol more ammonia than nitrogen.
nitrogen would produce 7.5 mol more ammonia than hydrogen.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Questions

Social Studies, 22.09.2021 19:10

History, 22.09.2021 19:10

Mathematics, 22.09.2021 19:10


History, 22.09.2021 19:10

Mathematics, 22.09.2021 19:10


Mathematics, 22.09.2021 19:10


English, 22.09.2021 19:10

Mathematics, 22.09.2021 19:10

English, 22.09.2021 19:10

World Languages, 22.09.2021 19:10


Mathematics, 22.09.2021 19:10

Mathematics, 22.09.2021 19:10

English, 22.09.2021 19:10

History, 22.09.2021 19:10

Mathematics, 22.09.2021 19:10

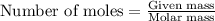
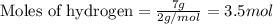
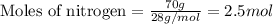
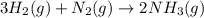
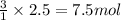 of nitrogen gas.
of nitrogen gas.

