Chemistry, 07.10.2019 03:30 jacobp0712
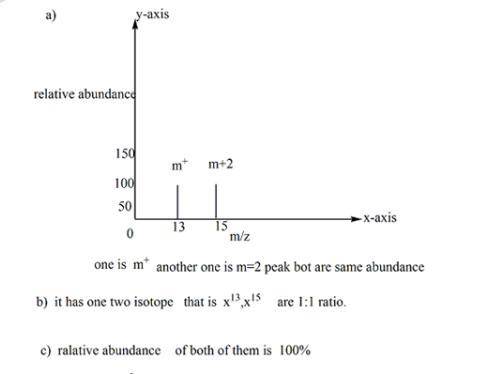
Suppose the mass spectrum of a hypothetical monatomic element x contains a signal at mass number 13 and another of identical height at mass number 15.
a. sketch the mass spectrum. make sure each axis is properly labeled.
b. how many isotopes are present? why?
c. what are the fractional abundances of the isotopes? why?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
What phase of matter has particles that are held together but can flow past each other and takes the shape of a container, filling it from the bottom up?
Answers: 1


Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
Suppose the mass spectrum of a hypothetical monatomic element x contains a signal at mass number 13...
Questions



Mathematics, 21.07.2019 16:30



History, 21.07.2019 16:30

Mathematics, 21.07.2019 16:30

Health, 21.07.2019 16:30










Chemistry, 21.07.2019 16:30