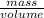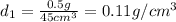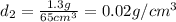Ascientist compares two samples of white powder. one powder was present at the beginning of an experiment. the other powder was present at the end. she wants to determine whether a chemical reaction has occurred. she finds that neither sample bubbles or dissolves in water. she measures the mass and volume of the solids. sample one has a volume of 45 cm3 and a mass of 0.5
g. sample two has a volume of 65 cm3 and a mass of 1.3
g. what should the scientist conclude?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
Ascientist compares two samples of white powder. one powder was present at the beginning of an exper...
Questions



Mathematics, 08.05.2020 10:57




Mathematics, 08.05.2020 10:57



Health, 08.05.2020 10:57


Mathematics, 08.05.2020 10:57



English, 08.05.2020 10:57

Mathematics, 08.05.2020 10:57


English, 08.05.2020 10:57

History, 08.05.2020 11:57

Physics, 08.05.2020 11:57