Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
You know the right answer?
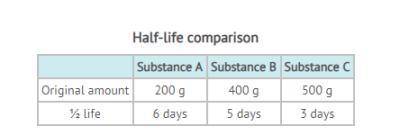
From the table of data, substance C will have the least of the original amount remaining after 30 da...
Questions

Biology, 18.07.2019 10:30


Mathematics, 18.07.2019 10:30

History, 18.07.2019 10:30

Mathematics, 18.07.2019 10:30


Mathematics, 18.07.2019 10:30





Mathematics, 18.07.2019 10:30






Mathematics, 18.07.2019 10:30

Biology, 18.07.2019 10:30